Nobel Prize in Chemistry in 2009 was awarded to three outstanding scientists “for studies of the structure and function of the ribosome”. They are Ada Yonath, Thomas A. Steitz and Venkatraman (Venki) Ramakrishnan. Unfortunately, Thomas A. Steitz from Yale University, USA died in 2018.
I was introduced to the work of Prof. Ada Yonath before she was awarded with Nobel Prize through one of her collaborators and her published papers.
My PhD thesis at The University of Manchester, United Kingdom was related to the investigation of macrolide antibiotics as anti-bacterial and potential anti-malarial agents. One of my tasks for the thesis was to investigate the interactions of bacterial ribosomes with macrolide antibiotics using TRNOESY NMR technique. This part of the work brings me personally a lot of joy and the excitement. Before me, many Jill Barber’s PhD students were working on the similar problems. Therefore, I was really excited and happy when one day my colleagues at Manchester informed me that some scientists got the Nobel Prize for their pioneering works on the ribosomes.
Venki Ramakrishnan was born in India in its state called Tamil Nadu, moved to Vadodara (Baroda) in Gujarat at the age of three, and did an undergraduate study in physics at Maharaja Sayajirao University of Baroda, India and PhD study in physics at Ohio University, USA. Then he spent two years studying biology as a graduate student at University of California, San Diego, USA. Among many honours, I would like to point out that he is the president of the Royal Society of Great Britain. Currently he is doing his research within his research group at MRC Laboratory of Molecular Biology in Cambridge, United Kingdom.
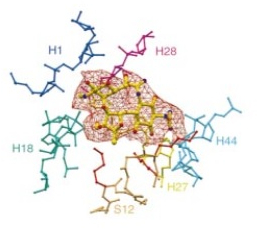
Chemical structure of streptomycin, showing
interactions of the various groups with specific residues of the ribosome.
Reprinted by permission from Copyright Clearance Center: Springer Nature, Nature, Functional insights from the structure of the
30S ribosomal subunit and its interactions with
antibiotics, Andrew P. Carter et al., 2000
Dr. Ramakrishnan agreed to answer several questions for the blog of the journal Chemia Naissensis related to his professional life.
- You were born in India in the family of scientists and chose to study physics and then you obtained PhD in physics, but you turned your scientific interest later to biology. Who was the influencer? What took you more closely to biology?
I found research in physics quite difficult, in the sense it was hard to figure out how to make major breakthroughs in what was a mature field. On the other hand, biology had been exploding for the previous few decades, and every issue of Scientific American was filled with major breakthroughs. It seemed a good time to move into biology. I was influenced by articles in Scientific American as well as various books, including The Double Helix by James Watson.
- One of your area of research interests is action of antibiotics on ribosomes. What do you think about TRNOESY NMR technique as a tool for the investigation of interactions of antibiotics and ribosomes? And what you can say about X-ray and cryo-electron microscopy in the investigation of those interactions? Can you tell us about advantages and disadvantages of the mentioned techniques regarding the investigation of the interactions of antibiotics and ribosomes?
I am not familiar with TRNOESY, but generally NMR has had a limited impact on our understanding of antibiotic action on the ribosomes. Historically, the major impact has come from biochemical methods and from x-ray crystallography, where one can directly visualize in detail the interactions of antibiotics in situ in the ribosome. The problem with crystallography is you need a significant quantity of material and you also need to get crystals that diffract well. However if you have good crystals, determining antibiotic structures is straightforward and can be done rapidly. Recently, cryoEM has superseded crystallography for the structure of ribosomes. However, each new antibiotic means determining the structure essentially again, rather than just doing a difference Fourier. Nevertheless, I suspect cryoEM will essentially take over much of the structural biology of ribosomes.
- As a famous and successful scientist born in India, do you give lectures and help scientific institutions in India?
In the past few years, I have visited India once a year (or sometimes once every two years). On these visits, I spend a significant fraction of time visiting institutes and giving lectures, as well as talking to scientists and students.


