Like plastics in the twentieth century, graphene is a material that has marked the 21st century. Scientists have theorized about graphene for decades. Now, researchers from around the world are teaming up to discover and learn every bit of information regarded to this supermaterial.
Origins
In the 16th century, a graphite mine was found in Cumberland, England. The mineral appeared to be an incredible material that could be used to forge weapons and tools because the melting-metal temperature didn’t have any effect on it. Later, in the year of 1794, Volta enlisted graphite in the Volta’s list of conductors. As a conductor of electricity, graphite was apparently to be considered as a metal.
In the first half of the twentieth century, Philip Russell Wallace was investigating the properties of graphite. He stated that it is composed of layers of the two-dimensional structures, which was later named graphene.
Predecessors
In 1889, Hughes and Chambers claimed a patent in which they introduced a method of manufacturing carbon fibers by using water vapor. In the year of 1953, Davis, Slawson, and Rigby used electron microscopy to spot some—larger than 100 angstroms (0.1 nanometers) in thickness—carbon fibers which rose from the action of carbon monoxide on iron oxide at the temperature of 450 Celsius degrees as they described in their paper published in Nature. In 1985, Smalley, Curl, and Kroto discovered fullerene, a sphere-shaped carbon macromolecule with the molecular formula C60. And six years later, a Japanese physicist, Sumio Iijima, used arc-discharge evaporation method to discover helical microtubules of graphitic carbon.
Graphene Today
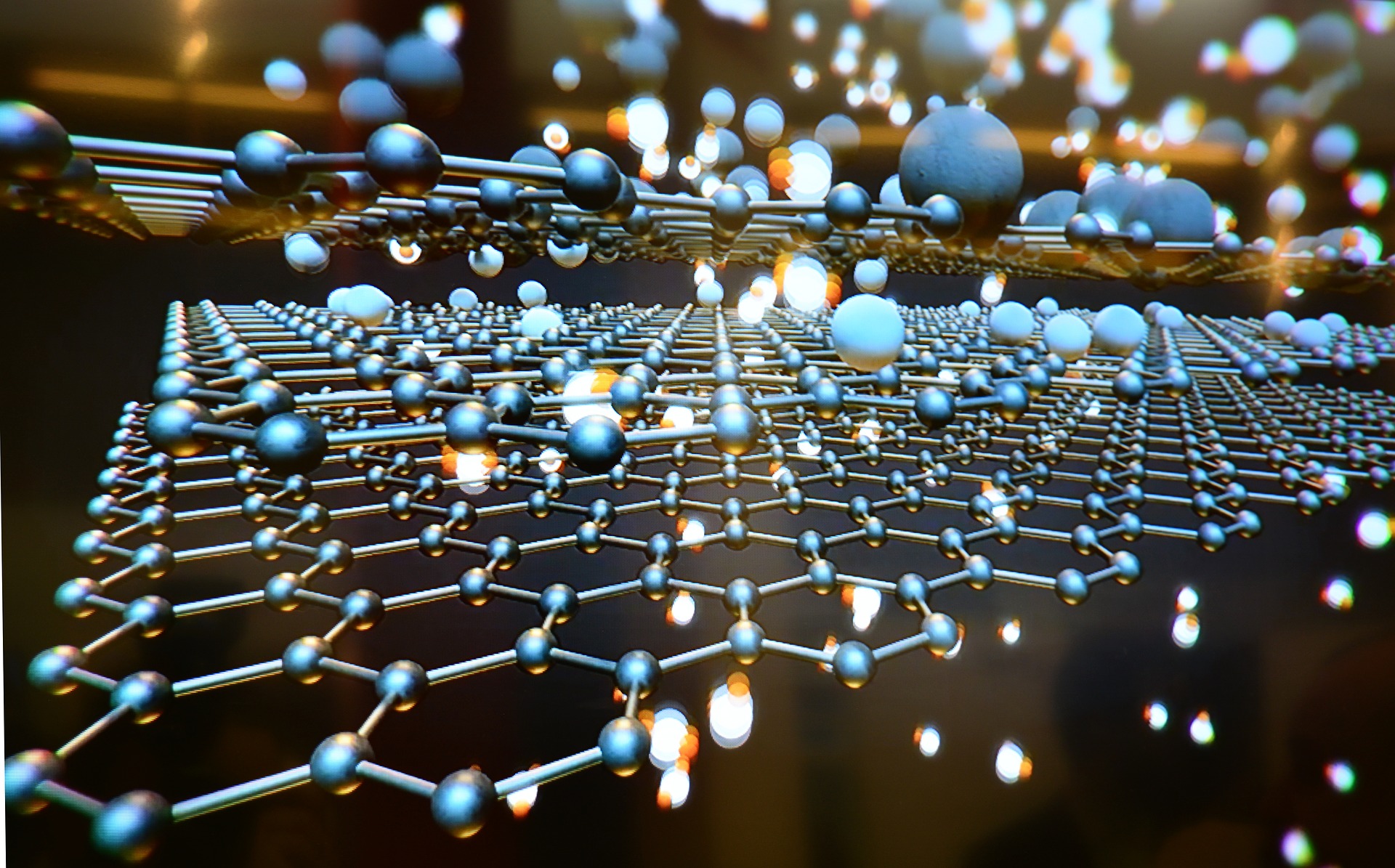
Graphene is an allotrope of carbon consisting of sheets that have the thickness of one atom. All carbon atoms are sp2 hybridized and arranged in a honeycomb pattern. Multiple graphene sheets stacked on top of each other are regarded as multi-layer graphene, up to the point where the material becomes graphite.
In 2004, Andre Geim, along with the Post-doc fellow Konstantin Novoselov, studied the properties of graphite and its derivatives. He suggested Novoselov to investigate the graphite remained on the adhesive tape, which is used for polishing graphite samples. Novoselov, then, found single-layered stable graphene, which was previously thought to be impossible to get. Because of the remarkable discovery of a simple and cheap method of obtaining single-layered graphene, in 2010, these two scientists won a Nobel Prize.
Graphene has extraordinary mechanical, optical and electrical properties, and hence this material is widely applicable in technology today. Here is the list of some properties of this amazing material:
- Weighs just 0.77 milligrams per square meter.
- Transparent – absorbs only 2.3% of visible light.
- Since it is a single 2D sheet, it has the highest surface area of all known materials.
- Graphene is flexible and the most stretchable crystal – up to 20% of its initial size without breaking.
- Highly impermeable – even helium atoms cannot go through graphene sheet.
- Amazing electrical and thermal conductivity properties.
- Graphene is quite an inert material despite the fact that all of graphene’s atoms are exposed to the environment.
- Antimicrobial properties allow this material to be used in wound dressing.
Project: Graphene
In 2013, the European Commission announced the winners of a multi-billion euro competition of Future and Emerging Technologies. The winning Graphene and Human Brain initiatives are set to receive one billion euros each, to deliver 10 years of world-beating science at the crossroads of science and technology.
The graphene initiative has a mission to connect researchers from 23 EU Member States and around 140 research institutes to contribute with their research in medical applications of graphene (e.g., artificial retina implants, biosensors, and biomarkers), long-lasting batteries, flexible LCD screens, etc.
In a 2014 study published in Nature, a Nobel laureate, Andre Geim, and his colleagues found that monolayered graphene and boron nitride are permeable to thermal protons under ambient conditions. These findings suggest that these materials can be used in the development of highly efficient proton exchange membranes.


Pingback: Interview with Professor Sir Konstantin S. Novoselov - Chemia Naissensis
I could not refrain from commenting. Perfectly written!| а
My family members every time sayy that I am killing my time here at web, however I know I am getting know-how everyday
by reading such pleasant content.
Your mode of telling everything in this paragraph is
actually good, every one bee capable of without difficulty be
aware off it, Thanms a lot.